CHAPTER
1 LESSON 6
Before you begin
the lesson, take the quiz.
CLICK
HERE for the quiz.
Your assignment is
to read the notes below and answer the questions that follow.
DIMENSIONAL ANALYSIS
You will recall from earlier lessons that a
system of measurement is based on a few selected physical quantities—such as
length, mass, time, and temperature—that are measured in arbitrary units. All
other quantities are derived from the fundamental quantities and are measured
in derived units. Speed (or velocity) is a derived quantity. The average speed
of a moving object is defined as the ratio of the distance (length) traveled to
the elapsed time of travel.
speed = length
= l
time t
Suppose an
object travels 576 m in 20.0 s. Its average speed will be
speed = l = 576 m
= 28.8 m/s
t 20.0 s
Note: Speed
is expressed in dimensions of length/time.
Based on the definition of speed, its
dimensions are length/time, or l/t.
Observe that the dimensions of speed are also specified in terms of the
derived unit m/s. Speed can be expressed
in any derived unit that represents its
dimensions of length/time. Speed cannot be expressed in any derived unit
that does not represent its dimensions of length/time. This concept is
fundamental in computations involving the dimensions of physical quantities.
A measurement is expressed as a significant
number of some kind of dimensional unit: 12.5 g, 6.72
cm, 10.0 s, 42.1°C, 0.09 g/L, 28.8 m/s. For a mathematical equation to
represent the correct solution to a problem, the equation must be dimensionally
correct. That is, both sides
of the equation must have the same dimensions.
For example, density is defined as the ratio of mass/ volume and has the dimensions of mass/length3.
The dimensional unit can be g/cm3.
If,
D = m then, g = g
V cm3 cm3
m = D
x V then, g = g x cm3 and g = g
cm3
V = m then, cm3 = g
D
g/cm3
or,
cm3 = g x cm3 and cm3 = cm3
g
Note that terms to both numerator and denominator are “canceled” by dividing both by the common term.
Dimensional analysis is a systematic way of solving numerical problems by the conversion of units. Frequently, your data will be recorded in one unit, but it will be necessary to do the calculation using a different unit of measurement. In the second example above, we are given cm3 and we need grams. So we will use dimensional analysis to be able to cancel the cm3 leaving grams. Likewise, in the third example, we want to cancel the grams and end up with cm3. Thus dimensional analysis allows us that step.
This
means you should multiply the number you wish to convert by a conversion factor
to produce a result in the desired unit.
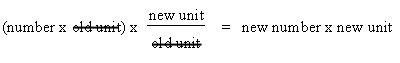
This way, the units in the denominator
cancel the units of the original data, leaving the desired units.
Observe that
dimensional units common to both numerators and denominators are
"canceled" and removed from the expressions in the same familiar way
as numerical factors.
Only
identical units cancel, as the above
operations illustrate. If mass is given in gram units in the numerator of an expression
and in kilogram units in the denominator, the units do not cancel directly. For
the mass units to cancel, either the measurement in grams must be converted to
kilograms, or the measurement in kilograms must be converted to grams. A valid conversion factor that can be introduced
into the expression for the purpose of making the desired unit conversion is
needed.
Metric
units are related as powers of 10 by the system of prefixes. One kilogram is
exactly one thousand grams; one centimeter is exactly one one-hundredth meter;
one second is exactly one thousand milliseconds. Such a unit relationship can
be expressed as a fraction and inserted into an equation as a con-version
factor. The form of this conversion factor must allow for the cancellation of
the unit to be converted and the retention of the unit required.
Suppose a length / is measured to three
significant figures as 1.30 m. How is / expressed in centimeters? By definition, 1 m is exactly 100 cm. A conversion factor
with the cm unit in the numerator (unit required) and the m unit in the
denominator (unit to be canceled) can be assembled.
In this
example, we are given a measurement of 1.30 m.
And we want to put the answer in cm.
We would use the dimensional analysis.
_
correct l = 1.30 m x 100 cm = 130
cm
m
Note we used the relationship of 1 m to 100 cm.
This enabled us to set up the problem so we could cancel the meters and
end up with cm.
-
The result, 130 cm, remains precise to three significant figures.
Had the conversion factor been assembled in the
inverted form, the meter units would not cancel, the answer unit would not be
in centimeters, and the
numerical result would not be a correct answer.
(incorrect) / = 1.30 m x m = 0.0130
m2
100 cm cm
Compare these two examples. Both forms of the conversion factor are valid expressions. The second
form, m/100 cm, however, is the conversion factor for converting centimeters to
meters. Observe that
m x cm = cm and
cm x m = m
m cm
The conversion
factor used in the first (correct) example not only yields the intended unit in
the answer hut also sets up the arithmetic computation that gives the correct
numerical answer.
These simple examples of the use of conversion factors reveal an
important strategy for solving problems involving measurements and their
dimensional units. In any
numerical equation, the units associated with the various quantities are
treated algebraically and are canceled, combined, etc., just like factors in
the equation.
When the numerical expression for the solution to a problem has been
assembled, the units in the expression should be "solved" for the
answer unit before the arithmetic is done. If the answer unit is dimensionally
correct for the physical quantity required, such as g/cm3 for density or m/s for speed, this
indicates that the arithmetic computation should yield the correct numerical answer. On the other hand, unit operations that yield an incorrect answer unit signal that the expression is incorrect and cannot give a correct answer to the problem.
This first unit-operations step
is a simple and rapid analysis of the dimensional character of the solution
setup for the problem. It
is the key step in the problem-solving technique called dimensional analysis.
This technique is often called the factor-label method. It is the method used in this course.
Later, the factor-label will be extended to include chemical formulas in the
unit-operations step of problem solving.
Remember, the factor-label method uses unit operations to set up the arithmetic in problem solutions.
The following Sample
Problems illustrate this factor-label method.
Problem
1
A chemistry student was asked to determine the density of an irregularly shaped
sample of lead, but was not supplied with any measurement data.
Solution:
The
student measured the mass of the lead on a "centigram" balance as
49.33 g. Recalling from General Science that a solid displaces its own volume
in a liquid, the student immersed the lead in water contained in a cylinder
graduated in 0.1-mL divisions. The sample displaced 4.35 mL
of water, the 0.05 mL being estimated.
Because the volume of a solid is
normally expressed in cubic measure, the measured volume was converted to cubic
centimeters.
Since 1 mL = 1 cm3
4.35 mL x 1 cm3 = 4.35 cm3
mL
Again, we
wanted the answer in cm3, but we were given mL
in the problem. So we used the conversion factor of 1 mL
being equal to 1 cm3 This allowed us to set up the problem
so that we could cancel the mL’s and get the cm3
By
definition: D = m, = 49.33
g = 11.34 g/cm3
V 4.35 cm3
The
multiplication/division rule for significant figures indicates an answer to 3
significant figures in this computation. Therefore, the result is rounded off
to 11.3 g/cm3.
A more
efficient solution setup for this problem is
D =
m,
= 49.33 g = 11.3 g/cm3
4.35 mL x 1 cm3/mL
|
|
Problem 2
_
Determine the concentration of table salt (sodium chloride) in grams of salt
per gram of solution, when 400 mg
_
of the salt is dissolved in 100 mL of water at 60 °C.
Solution:
The problem requires that the solution concentration be expressed in
grams of salt per gram of solution. Therefore, the solution concentration is derived
from the ratio: mass salt/mass solution. The mass of the solution is the sum of
the mass of salt dissolved and the mass of water used. The volume of water at
60 °C is ,
known, but its mass is required. Density relates the mass and volume of
a material. The density of water at 60 °C is listed in Appendix (Density of
Water Chart—Click here). Locate this
table and verify that the density of water at 60 °C is 0.983 g/mL.
By definition: D = m
V
Solving for m: m = D x V
_
Substituting: m = 0.983 g x 100 mL water
= 98.3 g water
mL
Observe that the unit operations yield the correct
answer unit for the mass of water.
The mass of
salt is given in milligrams. As
milligrams and grams cannot be added, milligrams of salt must be converted to
grams.
By
definition: 1 mg = 0.001 g
_
400 mg
salt x
0.001 g = 0.400 g salt
mg
Observe that
the factor 0.001 g/mg is exact.
mass of
solution = 0.400 g salt + 98.3 g
water
mass of solution = 98.7 g solution
solution
concentration = mass salt =
0.400 g
mass solution
98.7 g solution
solution
concentration = 0.00405 g salt/g solution
Here is another way to do
the same problem.
In this
example, the complete solution setup is developed in literal terms (without
number values) as an algebraic equation. Measurement values are then
substituted, and the indicated unit operations are performed to verify the
correct answer unit—grams salt per gram solution. It is an efficient method and
a productive approach to the problem-solving process.
solution
concentration = mass salt = mass
solution = ms
mass solution msoln
But, msoln = mass salt + mass water = ms
+ mw
And, mw =
density of water x volume of water = Dw x Vw
Therefore, soln
conc
= ms
[ms + (Dw x Vw)]soln
_
soln conc = _ 400 mg salt x 0.001 g/mg _
(400 mg x 0.001 g.mg + 0.983 g/mL x 100 mL)soln
soln conc = 0.400 g salt = 0.400 g salt
(0.400 g + 98.3 g) soln
98.7 g soln
soln cone
= 0.00405 g salt/g soln
|
|
PRACTICE PROBLEMS
1. Using a
platform balance, the mass of an irregular block of iron is found to be 280.2
g. The iron was immersed in water and found to displace 35.6 mL of the water. Determine the density of the iron.
2. Determine
the concentration of a solution in grams of salt per gram of solution when the
solution is prepared by dissolving 10.2 g of a certain salt in 500 mL of water at 50 °C.
Click here to see solution to problems